A Heart Under The Microscope
How organs on chips could revolutionize medical research
In 1537, Swiss physician Paracelsus came up with a method to craft a tiny model human: fill a gourd with human semen and put it in the womb of a horse to putrefy. The resulting transparent form must be fed with human blood for 40 weeks and kept warm by the horse, after which point a miniature child should grow.
History does not record if Paracelsus’ attempts were successful, and the lack of miniature children running around the world today suggests they probably weren’t. But our fascination with the idea of creating model people—simplified versions of our human ecosystems—has endured. We long to make computer brains, synthetic hearts, to watch full-fledged organs grow in labs. The instinct to simulate is part of our instinct to study and understand, and biological physicists continue to pursue the dream of model humans, with systems that perform like our own.
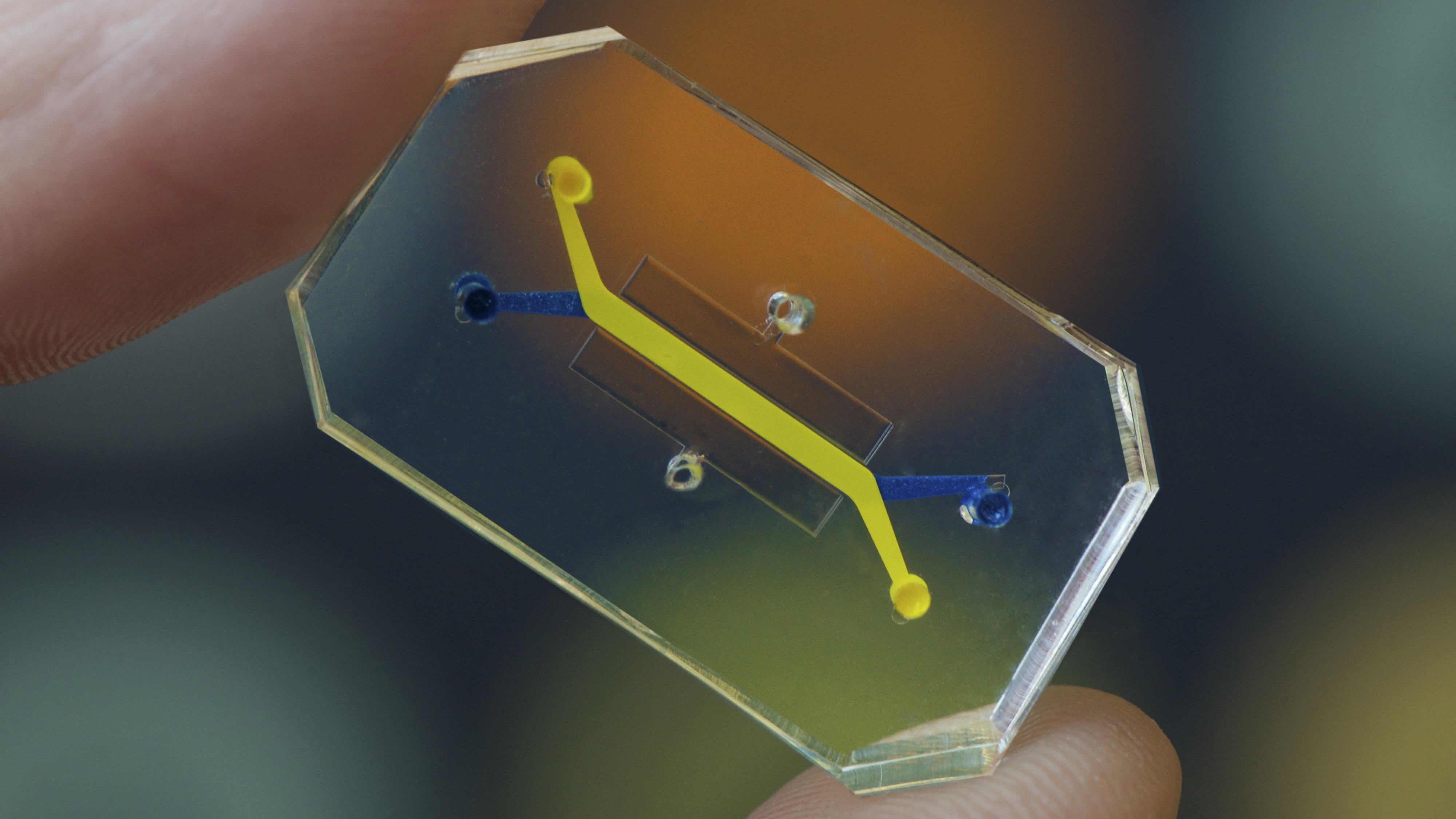 Image credit: Wyss Institute at Harvard University
Image credit: Wyss Institute at Harvard University
Testing on rats is the classic way we’ve simulated human biology. We share a large amount of genetic material with all mammals, including rats, and almost all human genes known to be associated with diseases have counterparts in the rat genome. But there are also major differences—circadian rhythms play a big role in pharmacology and toxicology, and rats are nocturnal.
Another alternative is to test drugs on parts of an organ—or the entire thing—in test tubes. This preserves the organ’s structure and layout, but it requires both careful handling and a regular supply of fresh organs—and when an organ is studied away from the body, it’s easy to miss interactions between it and other parts of the body. A drug that seems safe when tested on heart and liver cells separately might reveal a lethal transformation when it travels through a whole human test subject.

Today, the state of the art in attempts to model the insides of our bodies lies in the creation of “organs on chips.” They are generally a piece of glass, silicon, or polymer about the size of a computer memory stick.
Each chip can model a specific organ system, like the lungs, bone marrow, or intestines. Tiny tunnels run through the clear polymer chip, arranged to mimic normal—or even diseased—organ structures, so researchers can study what happens inside in great detail. These are abstractions: they won’t necessarily look like the real thing, in the same way that a subway map represents a city without actually looking like it. As long as they connect up in the same way as the real thing, they’re accurate.
Fluids and gases containing living human cells can then be piped down those channels, kept moving by mechanical or electrical stimulation and continually monitored by sensors or microscopes at key locations. Controlled, sterile conditions allow cell growth and other processes to happen without outside influence, and it’s even possible to simulate mechanical processes like beating hearts, or varying stresses like flexing muscles.
Make Your Own Organ-On-a-Chip
Select two inputs to try and find the four human organs that it's possible to replicate—or just experiment and see what you can create.

Acid
Blood

Food

Digested Food

Water

Waste
Oxygen
Organs on chips, more commonly called “microphysiological systems,” have vast potential, catching the attention (and financial backing) of not only the National Institutes of Health, but also of the Defense Advanced Research Projects Agency, or DARPA. Harvard’s Wyss Institute created the first successful organ chip, a miniature lung, in 2010, earning it a $37 million DARPA grant. DARPA, which has funded multiple microphysiological systems, says it’s interested in how these simulations of body parts can help defense departments create vaccines, respond to pandemics, or even prepare bioterrorism countermeasures as quickly as possible.

Enter John Wikswo, a founding director of Vanderbilt Institute for Integrative Biosystems Research and Education. Wikswo recently led a research team in creating an advanced model of the blood-brain barrier. The blood-brain barrier is a vital part of the brain system: it consists of specialized cells that surround the brain’s veins and arteries, and it acts a bit like security at a nightclub. Nutrients and other important things are allowed through, but substances like pathogens and toxins that will be harmful to the brain are stopped in their tracks.
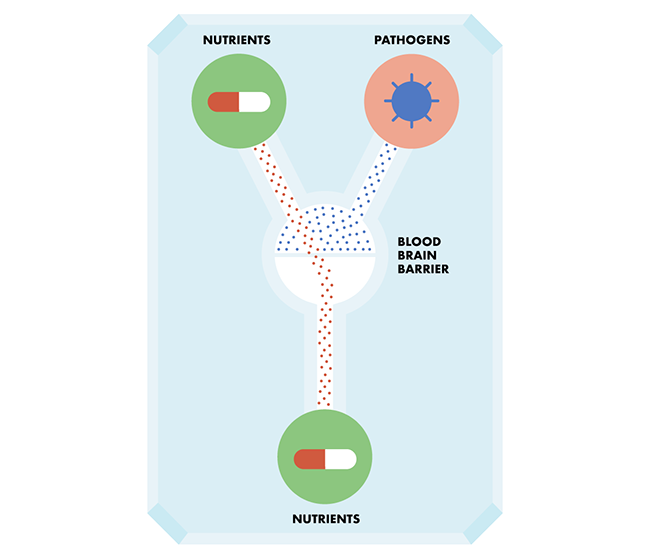
Wikswo’s team is using this model to study brain inflammation—which some neuroscientists call a “silent killer.” There is no pain involved in brain inflammation, but it contributes to conditions like Alzheimer’s and Parkinson’s disease, and may be behind a much wider range of problems, from poor cognition to schizophrenia and depression.
Drug makers can use the model to address the challenge of how to get drugs “past” this barrier and directly into the brain. “There are variations in melatonin, cortisol, and all sorts of other hormones that differ from organ to organ,” says Wikswo. “What we’re claiming is that organs on chips, and the technology for which they are developed, will allow you to re-create these variations in vitro.”
By getting human cells involved in drug development earlier in the pipeline, Wisko explains, new treatments can be brought to market more rapidly. Recent estimates place the cost of successfully developing a single new drug at $2.5 billion or more over the course of about 12 years. Advancements in efficiency or accuracy are crucial to lowering those costs.

But how well do these chips work together? In order to avoid the problem of missed interactions between different organs, it’s useful to be able to feed a drug through a complete biological system. If you patched together enough different organ chips, would you get a tiny human like the one Paracelsus envisioned? In a sense, yes, says Wikswo. These different human organ chips talk amongst themselves as they would in a “real” human body, and that makes it a safer way to quickly study new disease treatments than using rats or tissue samples alone.
While we may view these collections of glued-together chips as “little humans” for the purpose of medical modeling, they aren’t really “people”—no mind, no plasticity, no environment, no learning. Wikswo and his team are now working toward creating a brain on a chip with multiple regions—“collections of neurons of one flavor, talking through synapses to nearby neurons of another flavor,” he says. “We’re getting ready to put the neurons on electrode rays, so we can see how the neurons respond, in their electrical behavior, to drugs crossing barrier. We’re ratcheting up the realism, by including electrons.”
But a chip-based model of multiple brain systems would not be particularly intelligent—it would be too small. “A micro-brain is the size of a mouse brain, and mice are not particularly intelligent,” Wikswo says. “There will be people successfully building neural nets on electrodes in two or three dimensions, who can do computations—but that is not a fully-functioning brain. I don’t think anyone is talking about building a functioning brain.”
For now, recreating simple, specific human bodily systems seems to be the most valuable path to improving our drug development process and getting a better grasp of what’s happening inside of our bodies. “Genomics has brought us a very clear understanding of the individual parts,” Wikswo says. “But physiology is all those parts, working together.”

Credits: Interactive designed by Duncan Geere, Darren Garrett, and Eden Brackenbury, and created by The DataFace. Code licensed under a GPL-3.0 license and available on Github. Art by Eden Brackenbury.
For more on science/technology/culture from the Next team, sign up for our weekly newsletter:


